
Aurich Lawson | Getty Images
In the US, September is National Prostate Cancer Awareness Month. This feature highlights why catching prostate cancer early can be critical, and what researchers are doing to improve the odds of controlling the disease once it’s found.
Prostate cancer is a paradox. It has one of the highest early-stage survival rates of any cancer, yet it’s the second most common cause of cancer death in the US among people with a prostate (behind only lung cancer). Localized prostate cancer, only found in the organ itself, is highly curable. But once it becomes metastatic, spreading beyond the prostate, it is incurable and leads to death.
This makes studying it complicated. How do you understand something that is at once easy and impossible to cure? Researchers tackling the paradox are harnessing technologies like imaging, genetic sequencing, big data, and artificial intelligence to work toward changing outcomes for patients across the spectrum of cancer severity. From understanding what makes the cancer develop in the first place to identifying new drugs and new methods of treatment—each innovation is an opportunity to save lives. Here’s a look at just a few of the countless projects in progress around the world that could one day change the treatment landscape for prostate cancer.
Quantifying the paradox
The 10-year life expectancy for localized prostate cancer is around 98 percent. It’s remarkably curable. Many prostate tumors are so slow-growing and nonthreatening, they’re not even treated—they’re simply monitored to make sure they stay nonthreatening. “You’re more likely to die with prostate cancer than of prostate cancer,” says Dr. Isla Garraway, director of research in the Department of Urology at the University of California, Los Angeles (UCLA).
But once the cancer becomes metastatic, that prognosis changes completely. Treatments can improve a patient’s quality of life and increase their lifespan, but eventually the cancer will become resistant to all treatments and turn fatal. According to Dr. Yaw Nyame, an attending physician and assistant professor of urology at the University of Washington: “For most [prostate] cancers, when you are diagnosed because you have symptoms it’s oftentimes a sign that your disease is really advanced. If you have pain, or blood in the urine, or difficulty emptying your bladder, it’s likely you have advanced cancers.” In other words, the danger is compounded by the fact that there are no symptoms until it’s too late to cure.

/ Finding prostate cancer before it becomes metastatic is critical for managing the disease.
Meanwhile, the prevalence of the disease is staggering. About 1 in 8 people with a prostate (depending on who you ask) will be diagnosed in their lifetime. So far in 2021, the US has seen roughly 248,530 new cases and about 34,130 deaths.
Estimates of the percentage of metastatic cancers run from 5 to 10 percent, but the huge number of overall cases means that even the low-end estimate will ultimately mean a lot of cancer that resists treatment. And the CDC says the percentage is on the rise, which means it’s more important than ever for science to solve the problem of incurable metastatic prostate cancer.
Understanding the underlying causes
“For cancer researchers in general, we’re always seeking out the origins. Where does it start, what cells does it start in, how is it co-opting the processes of normal cells to evade the immune system or invade areas where it shouldn’t be?” says UCLA’s Dr. Garraway. “Just like other cancers, the whole idea is to understand the biology of the prostate tumors better so you can find the Achilles heel of that tumor.”
The challenge, Garraway tells Ars, is that for a long time, research into what causes prostate cancer growth was focused on the indolent (or slow-moving) versions of the disease that were curable. More surgeries removed slow-growing tumors, leaving researchers with more access to these tissue samples. Metastatic patients were less likely to undergo surgery because their cancers had spread to other parts of their body, so those samples were studied less often.
“For those 10 percent or so who are destined to have this metastatic disease, at least half of them already had spreading of their cancer at diagnosis,” she says. “They weren’t surgical candidates, so we weren’t capturing their tissues. And it’s a challenge to get enough tissue from a metastatic lesion.”
Recent advances in technology have changed that reality. Over the past decade, Garraway says next-gen genome sequencing technology has allowed scientists to classify and analyze minuscule amounts of tissue. Doctors can now use a small needle to collect “a very tiny little piece of tissue,” creating a paradigm shift in the study of lethal prostate cancer.
A look at prostate stem cells
It’s now possible to ask whether there are factors that mark high-risk tumors early on. Garraway compares these tiny samples of metastatic tumor cells to non-cancerous prostate tissue, hoping they can provide a clue as to the cause of the former’s aggressive behavior.
She does this by focusing on the stem cells normally found in the prostate, which can generate any cell type in this tissue (though, unlike embryonic stem cells, they can’t generate any other tissues). But stem cells show up in prostate tumors, too. Garraway hypothesizes that these prostate-specific stem cells might have features that make tumors more dangerous. Comparing the genes that are active in both prostate stem cells and cancers “gave us ideas about how these more aggressive cancers are co-opting benign stem cell traits. The stem cell’s primitive embryonic-type properties can facilitate infestation and spreading,” she says.
Because prostate stem cells can generate new glands, they’re really good at moving around and invading other parts of the body. “And they’re more hardy. They can survive things differentiated cells can’t survive,” Garraway says. “They’re resistant to radiation and chemotherapy, and they can survive damage to their DNA. We think activating these survival mechanisms supports tumor initiation.”
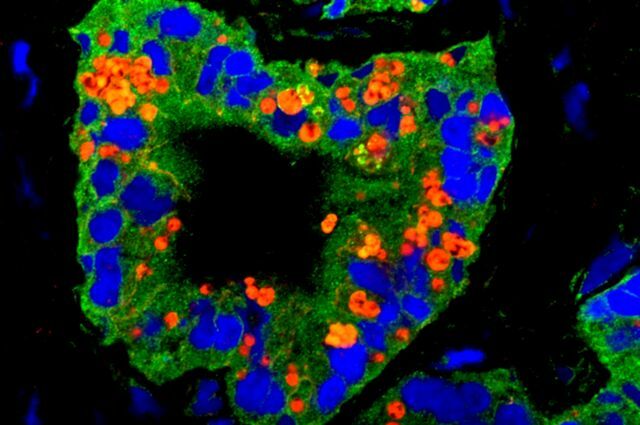
A prostate tumor, with green and red labels identifying the presence of proteins found in stem cells.
Garraway and her collaborators took healthy prostate tissue samples, sorting the cells by the different proteins they make, and identified if the cells have traits like the ability to regenerate prostate tissue. The results were compared to ones generated using the stem cells found in the metastatic tumor samples. “This is where it gets interesting,” says Garraway. It turned out that both benign tissue and the tumor cells expressed a protein called Keratin 13. That protein was very rare in most healthy tissues, but, ”we almost always saw [it] in biopsies that had metastatic disease,” she says.
Before this, the researchers thought Keratin 13 was what Garraway called a “run of the mill” protein that simply helped give a cell shape and stiffness. But they knew some keratins can also play a role in the signaling needed when cells migrate. “So now what we’re focused on is what it can do,” Garraway tells Ars. They’ve started by removing Keratin 13, which results in incapacitating the cells. “We were surprised cells that express Keratin 13 can no longer metastasize. It suggests it has a functional role in the metastatic process.”
This research is still in the very early stages. Garraway calls it “an example of how research and discovery is done, but it shouldn’t be considered a potential new therapy for now.”
Even if it doesn’t lead to treatments, the research she and her colleagues are doing will still contribute to the overall science of prostate cancer. “Our bigger focus is in understanding the tumor biology of these aggressive cells,” she tells Ars. “Prostate cancer that is metastatic is incurable. We have to understand who and why and how.”