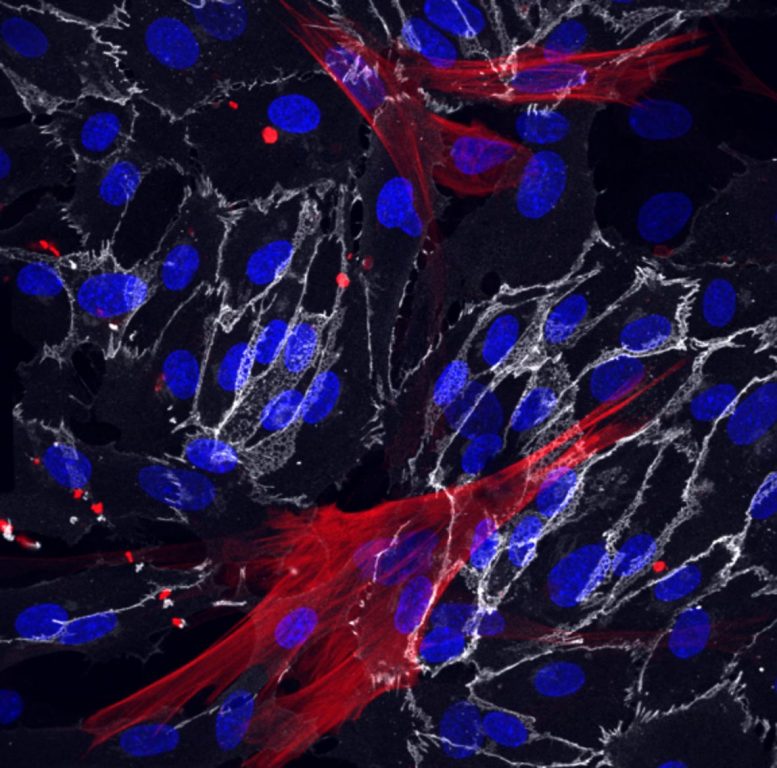
Skin fibroblasts were successfully reprogrammed into the smooth muscle cells (red) and endothelial cells (white) which surround blood vessels. The cells’ nuclei are shown in blue. Credit: Bersini, Schulte et al. CC by 4.0
Salk study is the first to reveal ways cells from the human circulatory system change with age and age-related diseases.
Salk scientists have used skin cells called fibroblasts from young and old patients to successfully create blood vessels cells that retain their molecular markers of age. The team’s approach, described in the journal eLife on September 8, 2020, revealed clues as to why blood vessels tend to become leaky and hardened with aging, and lets researchers identify new molecular targets to potentially slow aging in vascular cells.
“The vasculature is extremely important for aging but its impact has been underestimated because it has been difficult to study how these cells age,” says Martin Hetzer, the paper’s senior author and Salk’s vice president and chief science officer.
Research into aging vasculature has been hampered by the fact that collecting blood vessel cells from patients is invasive, but when blood vessel cells are created from special stem cells called induced pluripotent stem cells, age-related molecular changes are wiped clean. So, most knowledge about how blood vessel cells age comes from observations of how the blood vessels themselves change over time: veins and arteries become less elastic, thickening and stiffening. These changes can contribute to blood pressure increases and a heightened risk of heart disease with age.
In 2015, Hetzer was part of the team led by Salk President Rusty Gage to show that fibroblasts could be directly reprogrammed into neurons, skipping the induced pluripotent stem cell stage that erased the cells’ aging signatures. The resulting brain cells retained their markers of age, letting researchers study how neurons change with age.
In the new work, Hetzer and his colleagues applied the same direct-conversion approach to create two types of vasculature cells: vascular endothelial cells, which make up the inner lining of blood vessels, and the smooth muscle cells that surround these endothelial cells.
“We are among the first to use this technique to study the aging of the vascular system,” says Roberta Schulte, the Hetzer lab coordinator and co-first author of the paper. “The idea of developing both of these cell types from fibroblasts was out there, but we tweaked the techniques to suit our needs.”
The researchers used skin cells collected from three young donors, aged 19 to 30 years old, three older donors, 62 to 87 years old, and 8 patients with Hutchinson-Gilford progeria syndrome (HGPS), a disorder of accelerated, premature aging often used to study aging.
The resulting induced vascular endothelial cells (iVECs) and induced smooth muscle cells (iSMCs) showed clear signatures of age. 21 genes were expressed at different levels in the iSMCs from old and young people, including genes related to the calcification of blood vessels. 9 genes were expressed differently according to age in the iVECs, including genes related to inflammation. In patients with HGPS, some genes reflected the same expression patterns usually seen in older people, while other patterns were unique. In particular, levels of BMP-4 protein, which is known to play a role in the calcification of blood vessel, were slightly higher in aged cells compared to younger cells, but more significantly higher in smooth muscle cells from progeria patients. This suggests that the protein is particularly important in accelerated aging.
The results not only hinted at how and why blood vessels change with age, but confirmed that the direct-conversion method of creating vascular endothelial and smooth muscle cells from patient fibroblasts allowed the cells to retain any age-related changes.
“One of the biggest theoretical implications of this study is that we now know we can longitudinally study a single patient during aging or during the course of a treatment and study how their vasculature is changing and how we might be able to target that,” says Simone Bersini, a Salk postdoctoral fellow and co-first author of the paper.
To test the utility of the new observations, the researchers tested whether blocking BMP4 — which had been present at higher levels in smooth muscle cells developed from people with HGPS — could help treat aging blood vessels. In smooth muscle cells from donors with vascular disease, antibodies blocking BMP4 lowered levels of vascular leakiness — one of the changes that occurs in vessels with aging.
The findings point toward new therapeutic targets for treating both progeria and the normal age-related changes that can occur in the human vascular system. They also illustrate that the direct conversion of fibroblasts to other mature cell types — previously successful in neurons and, now, in vascular cells — is likely useful for studying a wide range of aging processes in the body.
“By repeating what was done with neurons, we’ve demonstrated that this direct reprogramming is a powerful tool that can likely be applied to many cell types to study aging mechanisms in all sorts of other human tissues,” says Hetzer, holder of the Jesse and Caryl Philips Foundation Chair.
The team is planning future studies to probe the exact molecular mechanisms by which some of the genes they found to change with age control the changes seen in the vasculature.
Reference: “Direct reprogramming of human smooth muscle and vascular endothelial cells reveals defects associated with aging and Hutchinson-Gilford progeria syndrome” by Simone Bersini, Roberta Schulte, Ling Huang, Hannah Tsai and Martin W Hetzer, 8 September 2020, eLife.
DOI: 10.7554/eLife.54383
Other researchers on the study were Ling Huang and Hannah Tsai of Salk. The work was supported by grants from the National Institutes of Health, the NOMIS Foundation and an AHA-Allen Initiative in Brain Health and Cognitive Impairment award made jointly through the American Heart Association and the Paul G. Allen Frontiers Group. Simone Bersini was supported by the Paul F. Glenn Center for Biology of Aging Research at the Salk Institute.
