
In mice, a vaccination strategy that uses an mRNA coronavirus vaccine injection followed by a nasal spray booster generates immune protection in the airways.
A new coronavirus vaccine guards one body part especially vulnerable to infection: the nose.
Dosing mice with a nasal spray booster recruited an army of immune defenders to both the nasal cavity, where coronaviruses typically enter the body, and the lungs, scientists report in a preprint posted January 26, 2022, on bioRxiv.org.
Made only of coronavirus spike protein, the vaccine is part of a one-two punch that could one day protect people from infection. Dubbed “Prime and Spike,” the strategy relies on an mRNA coronavirus vaccine injection that primes the immune system to recognize SARS-CoV-2, followed by a nasal spray vaccine that shores up defenses at the mucus membranes.
A nasal spray coronavirus vaccine booster helps protect mice from SARS-CoV-2. In this electron microscopy image, viral particles are shown as blue circles. Credit: CDC/ Hannah A Bullock; Azaibi Tamin
Such a strategy might offer a way to counter the waning effectiveness of current mRNA coronavirus vaccines, says study author Akiko Iwasaki, a Howard Hughes Medical Institute Investigator at Yale University.
Until now, scientists had not tested nasal vaccines on animals that already had some pre-existing immunity, says Jacco Boon, a virologist at Washington University School of Medicine in St. Louis who was not involved with the new work. “This paper is telling us that the intranasal booster induces a really good immune response in the nose and the lungs,” he says. “It’s a clever strategy, and I hope they test it in people.”
Guardians at the gates
Less than a year after SARS-CoV-2 surfaced in Wuhan, China, the United States had an mRNA vaccine ready to go. The technology, based on decades of research, proved safe and remarkably effective at preventing serious illness and death. But scientists now know the effectiveness of these coronavirus vaccines begins to drop in the months after the second dose – and increased protection afforded by a third dose may be temporary.
Though current mRNA vaccines work well at preventing severe disease in most people, Iwasaki says, they weren’t designed to prevent infection, so the virus can still skip from person to person. What’s more, vaccine hesitancy within the US remains high. In some regions, roughly a quarter of people surveyed said they would probably or definitely not receive a COVID-19 vaccine, according to an estimate from the US Centers for Disease Control and Prevention.
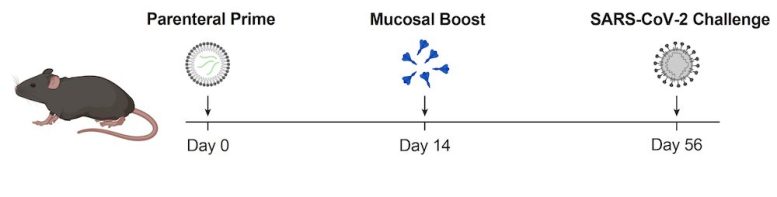
A new vaccine strategy tested in mice uses an mRNA coronavirus vaccine injection (Parenteral Prime) followed by a nasal spray spike protein coronavirus vaccine (Mucosal Boost). This approach protected mice from disease caused by exposure to SARS-CoV-2. Credit: T. Mao et al./bioRxiv 2022
Scientists have not untangled all the factors that contribute to vaccine hesitancy, but Iwasaki thinks having another option for vaccine delivery could be helpful. She started with something deceptively simple: just the full spike protein in solution. “There’s nothing else in the vaccine,” she says. “No adjuvants, no vector, no nothing. It’s off-the-shelf, kind of simple.”
And instead of aiming for the kind of broad, systemic immunity generated by the mRNA vaccines, the team kept their focus local – on the wet, mucus-producing tissue lining the nose and lungs. A nasal spray vaccine, they thought, could beef up immune security at these sites of viral entry in people who already have immune memory cells due to vaccines or infections. It’s like adding extra bouncers at the doors of a club – in theory, Iwasaki says, these “guardians at the gates” could banish any would-be viral invaders before they begin multiplying in the body. That would stop an individual’s infection and prevent the virus from spreading to others.
Prime and Spike
The most well-known nasal spray vaccine may be FluMist, an influenza vaccine that relies on weakened viruses to generate immunity. But it’s not available for everyone. FluMist is approved only for people 2 to 49 years old, and because it uses living viruses, it is not suitable for those with compromised immune systems.
That problem isn’t an issue for Iwasaki’s nasal spray coronavirus vaccine because it’s made of protein, not living virus. And unlike FluMist, Iwasaki’s spray doesn’t work on its own. It requires a partner vaccine to initially prime the immune system, like giving it a look at the virus’s mugshot.
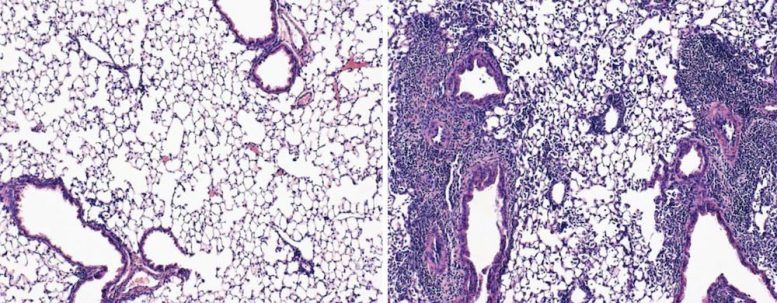
Mice exposed to coronavirus after an mRNA vaccination shot and a nasal spray booster had healthy lungs full of airspace (left). Mice that received only the shot had damaged lungs that showed signs of pneumonia (right). Credit: T. Mao et al./bioRxiv 2022
First, her team injected mice with one dose of the mRNA vaccine Comirnaty (the Pfizer-BioNTech COVID-19 vaccine). Two weeks later, the researchers squirted the spike protein vaccine into the animals’ noses. That spray rallied protective antibodies and immune cells to stand guard at the mucus membranes, Iwasaki explains. “These immune cells are waiting in the nose like ninjas,” she says. “They know what to look for because they’ve seen it before.”
The strategy protected the animals from the original SARS-CoV-2 strain, which causes COVID-19. Roughly 80 percent of mice that received only an injected vaccine containing SARS-CoV-2 spike mRNA died in the two weeks following coronavirus exposure, the team showed. In contrast, all the mice that received both the injected vaccine and the nasal booster survived.
“Not only do they survive, but their lungs are just so clean,” Iwasaki says. Under the microscope, the scientists saw healthy lung tissue, which looks like a bunch of overinflated balloons.
Iwasaki’s team also tested a second type of nasal booster, made with mRNA sheathed in biodegradable polymers. That worked as well as the spike protein vaccine, illustrating the new strategy’s versatility, she says. A nasal booster may even work in unvaccinated people previously infected with coronavirus. Iwasaki thinks it’s possible that an infection could effectively prime the immune system.
After testing the new vaccine in larger animals, Yale hopes to begin clinical trials in humans. The Prime and Spike strategy has been licensed to Xanadu Bio, a start-up company that Iwasaki and others co-founded at Yale. Xanadu Bio will help form a partnership with pharmaceutical companies that could manufacture and test the nasal spray vaccine.
“We’re hoping that Prime and Spike can protect people against future coronavirus variants,” Iwasaki says.
Reference: “Unadjuvanted intranasal spike vaccine booster elicits robust protective mucosal immunity against sarbecoviruses” by Tianyang Mao, Benjamin Israelow, Alexandra Suberi, Liqun Zhou, Melanie Reschke, Mario A Peña-Hernández, Huiping Dong, Robert J. Homer, W. Mark Saltzman and Akiko Iwasaki, 26 January 2022, bioRxiv.
DOI: 10.1101/2022.01.24.477597
Akiko Iwasaki’s team’s work was supported, in part, by HHMI funding dedicated to collaborative research projects on SARS-CoV-2 and the disease it causes, COVID-19. HHMI is now launching a further investment into preparedness for future pandemics through the $250 million Emerging Pathogens Initiative (EPI). EPI is designed to support research projects from HHMI Investigators about fundamental research into emerging pathogens.