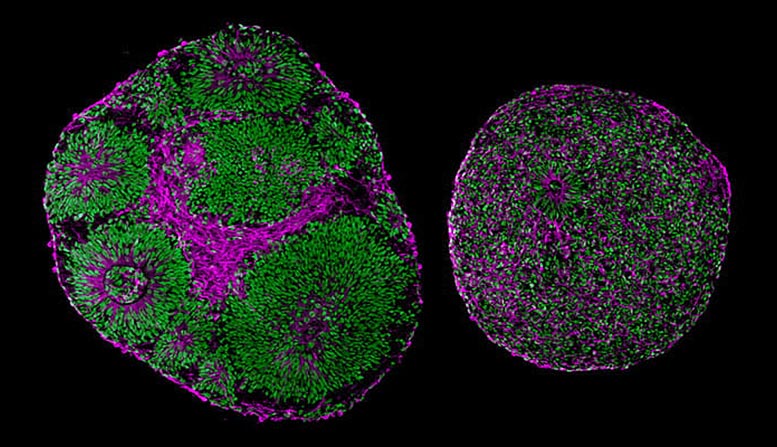
Microscopy images reveal significant differences in size and structure between brain organoids derived from a patient with Pitt-Hopkins Syndrome (right) and from a control (left). Credit: UC San Diego Health Sciences
University of California San Diego (UCSD) study uses lab-grown human brain tissue to identify neural abnormalities in Pitt-Hopkins Syndrome and test gene therapy tools.
In a study published on May 02, 2022, in the journal Nature Communications, scientists at the University of California San Diego School of Medicine used human brain organoids to discover how a genetic mutation associated with a severe form of autism disrupts neural development. Using gene therapy tools to recover the gene’s function successfully rescued neural structure and function.
Several neurological and neuropsychiatric diseases, including autism spectrum disorders (ASD) and schizophrenia, have been linked to mutations in Transcription Factor 4 (TCF4), an essential gene in brain development. Transcription factors regulate when other genes are switched on or off, so their presence, or lack thereof, can have a domino effect in the developing embryo. Still, little is known about what happens to the human brain when TCF4 is mutated.
To explore this question, researchers focused on Pitt-Hopkins Syndrome, an ASD specifically caused by mutations in TCF4. Children with the genetic condition have profound cognitive and motor disabilities and are typically non-verbal.
Pitt–Hopkins Syndrome (PTHS) is a rare genetic disorder characterized by developmental delay, epilepsy, distinctive facial features, and possible intermittent hyperventilation followed by apnea. As more is discovered about Pitt–Hopkins, the disorder’s developmental spectrum broadens to encompass difficulties with autism, anxiety, ADHD, and sensory disorders. It is linked to an abnormality within chromosome 18, specifically an inadequate expression of the TCF4 gene.
Existing mouse models of Pitt-Hopkins Syndrome fail to accurately mimic patients’ neural characteristics, so the UCSD team instead created a human research model of the disorder. Using stem cell technology, they converted patients’ skin cells into stem cells, which were then developed into three-dimensional brain organoids, or “mini-brains.”
Initial observations of the brain organoids revealed a slew of structural and functional differences between the TCF4-mutated samples and their controls.
“Even without a microscope, you could tell which brain organoid had the mutation,” said senior study author Alysson R. Muotri, PhD, professor at UC San Diego School of Medicine, director of the UC San Diego Stem Cell Program, and member of the Sanford Consortium for Regenerative Medicine.
The TCF4-mutated organoids were substantially smaller than normal organoids, and many of the cells were not actually neurons, but neural progenitors. These simple cells are meant to multiply and then mature into specialized brain cells, but in the mutated organoids, some part of this process had gone awry.
A series of experiments revealed that the TCF4 mutation led to downstream dysregulation of SOX genes and the Wnt pathway, two important molecular signals that guide embryonic cells to multiply, mature into neurons, and migrate to the correct location in the brain.
Due to this dysregulation, neural progenitors did not multiply efficiently and thus fewer cortical neurons were produced. The cells that did mature into neurons were less excitable than normal and often remained clustered together instead of arranging themselves into finely-tuned neural circuits.
This atypical cellular architecture disrupted the flow of neural activity in the mutated brain organoid, which authors said would likely contribute to impaired cognitive and motor function down the line.
“We were surprised to see such major developmental issues at all these different scales, and it left us wondering what we could do to address them,” said first author Fabio Papes, PhD, associate professor at the University of Campinas and visiting scholar at UC San Diego School of Medicine, who jointly supervised the work with Muotri. Papes has a relative with Pitt-Hopkins Syndrome, which motivated him to study TCF4.
The team tested two different gene therapy strategies for recovering the functional gene in brain tissue. Both methods effectively increased TCF4 levels, and in doing so, corrected Pitt-Hopkins Syndrome phenotypes at molecular, cellular, and electrophysiological scales.
“The fact that we can correct this one gene and the entire neural system reestablishes itself, even at a functional level, is amazing,” said Muotri.
Muotri notes that these genetic interventions took place at a prenatal stage of brain development, whereas in a clinical setting, children would receive their diagnosis and treatment a few years later. Thus, clinical trials must first confirm whether a later intervention is still safe and effective. The team is currently optimizing their recently licensed gene therapy tools in preparation for such a trial, in which spinal injections of the genetic vector would hopefully recover TCF4 function in the brain.
“For these children and their loved ones, any improvements in motor-cognitive function and quality of life would be worth the try,” Muotri said.
“What is truly outstanding about this work is that these researchers are going beyond the lab and working hard to make these findings translatable to the clinic,” said Audrey Davidow, president of the Pitt Hopkins Research Foundation. “This is so much more than a stellar academic paper; it’s a true measure of what well-practiced science can accomplish to hopefully change human lives for the better.”
Reference: “Transcription Factor 4 loss-of-function is associated with deficits in progenitor proliferation and cortical neuron content” by Fabio Papes, Antonio P. Camargo, Janaina S. de Souza, Vinicius M. A. Carvalho, Ryan A. Szeto, Erin LaMontagne, José R. Teixeira, Simoni H. Avansini, Sandra M. Sánchez-Sánchez, Thiago S. Nakahara, Carolina N. Santo, Wei Wu, Hang Yao, Barbara M. P. Araújo, Paulo E. N. F. Velho, Gabriel G. Haddad and Alysson R. Muotri, 2 May 2022, Nature Communications.
DOI: 10.1038/s41467-022-29942-w
Co-authors include: Janaina S. de Souza, Ryan A. Szeto, Erin LaMontagne, Simoni H. Avansini, Sandra M. Sanchez-Sanchez, Wei Wu, Hang Yao and Gabriel Haddad at UC San Diego; Antonio P. Camargo, Vinicius M. A. Carvalho, Jose R. Teixeira, Thiago S. Nakahara, Carolina N. Santo, Barbara M. P. Araujo and Paulo E. N. F. Velho at the University of Campinas.
This work was funded, in part, by the National Institutes of Health (grant R01 MH123828), the Pitt Hopkins Research Foundation, the Sao Paulo Research Foundation (grants 2020/11451-7, 2018/03613-7, 2018/04240-0) and the U.S. Department of Energy Joint Genome Institute (DE-AC02-05CH11231).
Disclosures: Alysson R. Muotri is the co-founder of and has an equity interest in TISMOO, a company dedicated to genetic analysis and human brain organogenesis.